Nobel Prize in Chemistry 2025: What was it awarded for?
Header image: Niklas Elmehed © Nobel Prize Outreach
Just a few days ago, a series of Nobel Prizes were awarded. Among which the Nobel Prize in Chemistry 2025, which went to Susumu Kitagawa, Richard Robson and Omar M. Yaghi. Of the university of Kyoto, Melbourne and California (Berkeley), respectively. I can’t blame you, however, if these names don’t say much to you and you’re wondering “What on earth did they do to win?”
According to the Royal Swedish Academy of Sciences, the Nobel Prize laureates “created molecular constructions with large spaces through which gases and other chemicals can flow”1. These materials can essentially stretch to make holes between the molecules holding it together. These holes could then be filled with numerous other materials, such as hydrogen gas or carbon dioxide. As a result, these materials could find application in a variety of fields.
The Nobel Prize in Chemistry 2025
- Introduction
- Metal-Organic Frameworks (MOFs)
- Synthesis of early MOFs
- What does the future of MOFs entail?
The media is already discussing it’s potential use in capturing CO2 out of the atmosphere thanks to the large cavities (“holes”) in this material. Even some electrically conductive versions have been developed, which could be used as semiconductors.
On top of that, hydrogen gas can easily be stored within it. Typically, hydrogen gas is useful in making ammonia, a vital ingredient in fertilizers, but it also has the highest specific energy of any fuel. (Meaning it can store more energy per unit of mass than any other fuel). However, hydrogen is painfully difficult to transport because it takes up a lot of volume. So transporting it means needing a lot of room and a small amount of it won’t give you a lot of energy. Turning it into a liquid makes this easier, but requires an incredible energy input to keep it from turning into a gas. Thus, as you can imagine, having a material to store it in proves as very useful (duh – they won a Nobel prize!).
But what is this mysterious material that seems to defeat all evil? And how on earth did these three Nobel Prize laureates make it?
Metal-Organic Frameworks (MOF)
In 1989, Richard Robson connected long “rods” of organic molecules to metal atoms, specifically those with tetrahedral and octahedral geometries.2 This metal atom would hold these long organic molecules in place, forming a crystal-like structure. In fact, a crystal gets its features from its network of carbon atoms. These carbon atoms each form four bonds with each other, leaving cavities of empty space in the molecular structure.
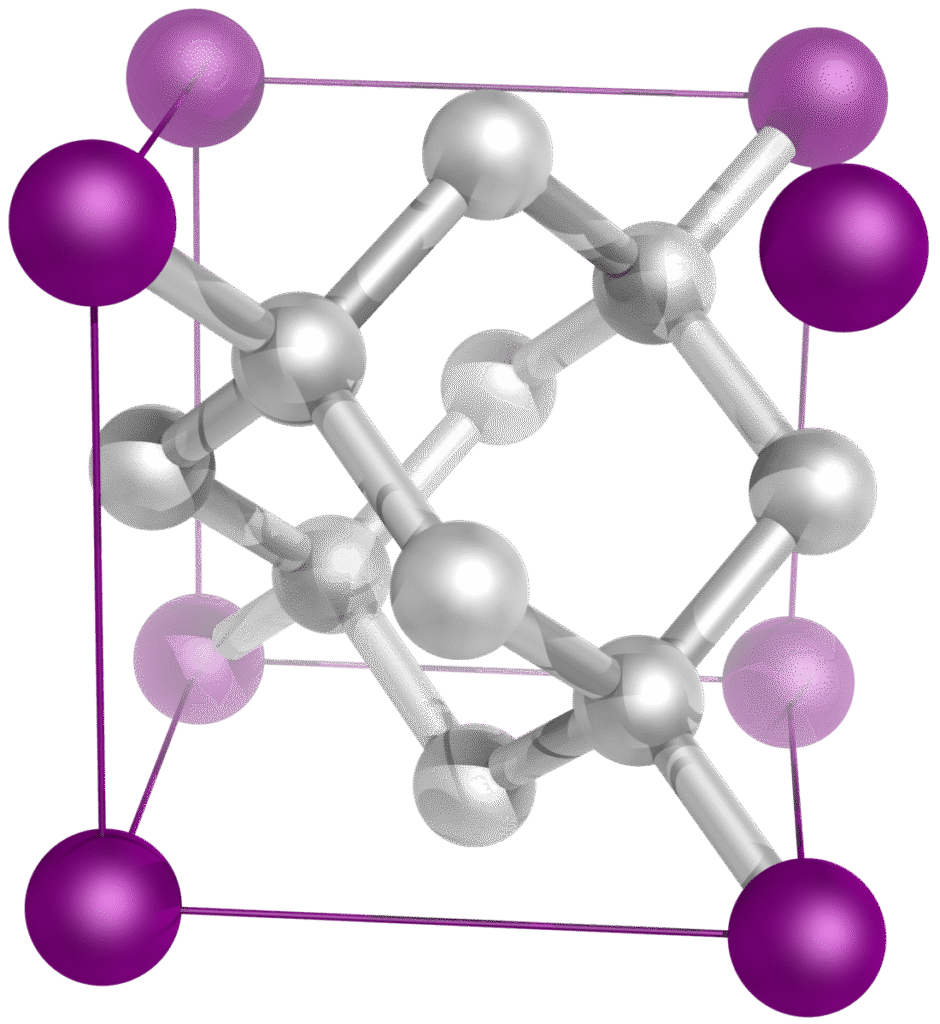
Robson originally carried out his experiment with copper ions Cu²⁺ and 4,4′,4″,4′”-tetracyanotetraphenylmethane (the organic molecule). 4,4′,4″,4′”-tetracyanotetraphenylmethane has four cyanide CN− groups, which, when paired with a metal, strongly attract and pull away the electrons of the metal. This type of molecule is thus also called an electron-withdrawing ligand. For more on ions and electrostatic attraction click here.
So as a result of the copper ion’s positive charge and the organic molecules’ negative charge, a molecule is formed with a metal center and “arms” of organic molecules extending from it (Figure 2). This reminds a bit of a diamond, just that instead of carbon atoms having four bonds with each other, strings of organic molecules form four (tetrahedral) or six (octahedral) bonds with a metal atom. This specific type of molecule is also called a metal coordination complex (in case you want to search it up!).
Synthesis of early MOFs
Since Robson’s synthesis of an early metal-organic framework (MOF), a lot of other types have been produced. For example, MOF-177 has especially large pores, where e.g. hydrogen can be stored. It has 7.1 weight percent (wt%), meaning it can store e.g. 7 grams of hydrogen per 100 grams of MOF-177. However, this is only achieved at very low temperatures (77K, or -196.15 °C) and high pressure (40 bar).3
However the creation of MOF-177 was only a later step in the development of metal-organic frameworks. The problem was that the molecule Robson created was very unstable. And thus, Yaghi, another one of the laureates, and his coworkers synthesized MOF-5, a much stabler form of MOF4. MOF can be heated to 300°C and doesn’t collapse when gas exits the structure. It is made of Zn₄O-carboxylate “nodes” (the metal part) connected to 1,4-benzodicarboxylic acid “arms”. MOF was also an exceptional breakthrough because it exhibits a high surface area to volume ratio.
What does the future of MOFs entail?
The Nobel Prize award in this area of Chemistry will, at least in my opinion, undoubtedly attract more attention to the study of MOFs. MOFs have found applications in anything from extracting CO2 from the atmosphere to harnessing water resources.5 They are and can become more useful in industry, such as in the separation or purification of chemicals. Many traditional separation techniques are energy-intensive. Yet MOFs provide a certain level of fine-tuning that can extract certain molecules from substances, and thus they could become interesting for a number of companies. Perhaps soon many of the daily objects we use will contain MOFs.
If that truly is the case, then I hope I have adequately informed and prepared you for a future with MOFs.
back to top ↑
- Nobel Prize in Chemistry 2025. NobelPrize.org. https://www.nobelprize.org/prizes/chemistry/2025/press-release/ ↩︎
- Hoskins, B., & Robson, R. (1989). Infinite Polymeric Frameworks Consisting of Three Dimensionally Linked Rod-like Segments. Journal of the American Chemical Society, 111(15). https://doi.org/10.1021/ja00197a079 ↩︎
- Rowsell, J. L. C., Millward, A. R., Park, K. S., & Yaghi, O. M. (2004). Hydrogen sorption in functionalized Metal−Organic frameworks. Journal of the American Chemical Society, 126(18), 5666–5667. https://doi.org/10.1021/ja049408c ↩︎
- Li, H., Eddaoudi, M., O’Keeffe, M., & Yaghi, O. M. (1999). Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature, 402(6759), 276–279. https://doi.org/10.1038/46248 ↩︎
- Selim, S. (2024, April 15). Metal-Organic Frameworks (MOFs) 2024-2034: Market, technology, and players: IDTechEX. IDTechEx. https://www.idtechex.com/en/research-report/metal-organic-frameworks/1004 ↩︎
2 thoughts on “Nobel Prize in Chemistry 2025: What was it awarded for?”