Can I make cookies out of potatoes? (theoretically)
Here is my grandmother’s recipe for butter cookies: 250g flour, 125g butter, 100g sugar, 1 egg, a dash of vanilla, half a teaspoon of baking powder, a sprinkle of salt. Mix, chill, roll and cut into shapes. I’ve eaten these cookies immeasurably often, and so, being curious, I wondered: is it possible to do the same thing… with potatoes?
You might’ve come across all kinds of special recipes that avoid using wheat flour. Gluten free cookies, almond flour brownies, rice flour pie crust… you get the point. But I don’t want to simply share another alternative baking recipe with you: I want to use chemically modified potato starch as a base for my cookies.
The plan is simple: extract starch from potatoes and turn it into simple sugars. Surprisingly, this is done more often than you think in industry. High fructose corn syrup, beer and other glucose syrups find innumerable applications in our everyday lives.
But to understand how we might make cookies from potatoes, we must first unpack what starch actually is, and what happens when we break it down.
- What is starch?
- Amylose and amylopectin
- What is starch used for and why?
- How starch breaks down into sugar and why it matters
- The experiment
For more on food chemistry, check out how milk is made into cheese.
What is starch?
Starch is found in many loved products, from cakes and tarts to peas and corn, and is considered a polymeric or complex carbohydrate, meaning it is made up of smaller components. It is the main component of many different flours, but its origins lie in nature: Starch is produced by the majority of green plants as means to store energy that is made during photosynthesis.
Starch is made up of many small sugar components, called glucose, which link together to form one long chained molecule. These long chained molecules are amylose and amylopectin, which are both made up of glucose. However, these two polymers of starch differ in their arrangement of glucose molecules.
Amylose consists of a linear chain of glucose molecules which are joined by glycosidic links, essentially atoms that join two glucose molecules together. On the other hand, amylopectin contains glucose molecules that arrange in branch-like structures. This difference, although it might seem unimportant, plays an important role in the consistency of starch and its chemical behavior. For example, pea starch can contain as much as 60% amylose, while most other starches contain 20-25%. As a result, pea starch has a remarkable stability to heat and gelling ability.
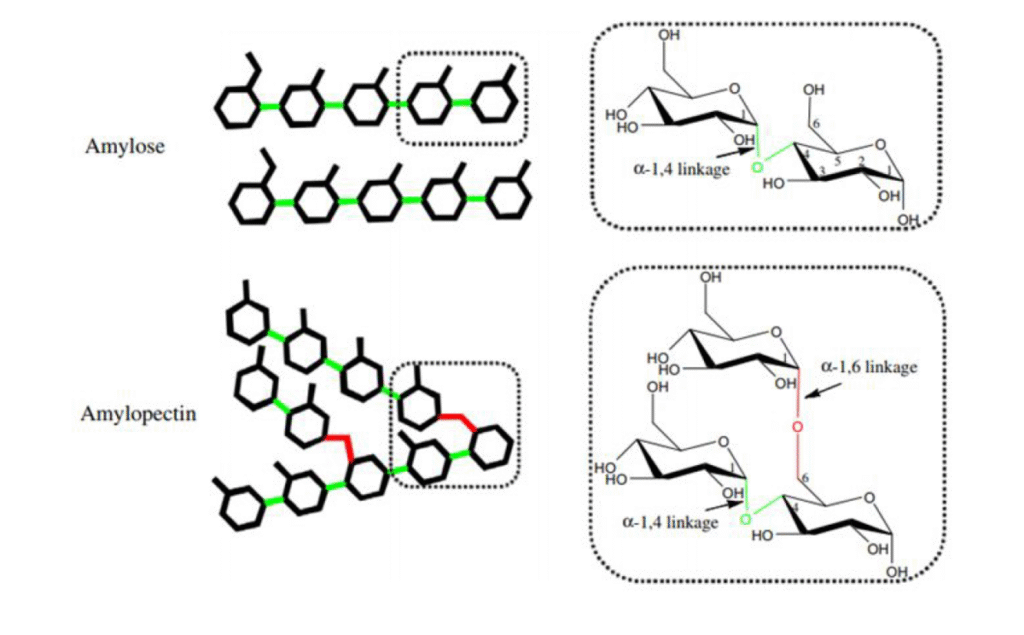
Amylose and amylopectin
Amylose
As the main components of starch, amylose and amylopectin are quite different to each other, nonetheless. As mentioned, amylose consists of a chain of glucose molecules, which are linked together by gylcosidic links. These glycosidic links connect carbons atoms 1 and 4 of two glucose atoms to each other. Hence, they are called 1,4-glycosidic links.
Glucose has some electronegative hydrogens, which form hydrogen bonds between chains. As a result, two or more linear amylose chains can twist around one another, forming a helical structure. This has not only the advantage of being more space-effective, but is also more resistant to enzymatic breakdown. Enzymes cannot access the chains as easily, and as a result, starch cannot be broken down easily, which leads to a lower spike in blood sugar and can make you feel more full.
Amylopectin
Amylopectin has an equally interesting 3D structure. A model for the molecule proposed by Robin et al1 suggests an arrangement where one chain branches off into different clusters, called the ‘cluster model’. As it turns out, some amylopectin chains are longer than others. So, the shorter chains are referred to as A chains, while the longer ones are called B chains. In each amylopectin molecule, there are also C chains, which contain a reducing end-group. In my opinion, the model makes the molecule look a bit like a family tree, as you can see below.
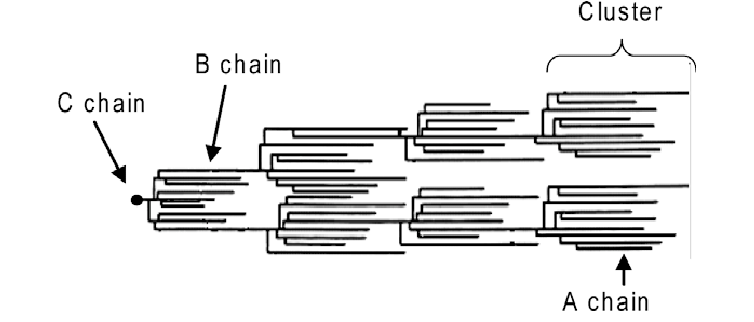
The C chain, which carries the reducing end, faces towards the middle of the starch granule, the hilum. From there on, all the other chains develop ‘outwards’. This forms regions with branch points, called amorphous regions, as well as regions without branch points: crystalline regions. This difference in structure affects enzymes: For example, regions with branch points are easier for enzymes in your body to unpack than crystalline regions, where amylopectins form helical structures.
What is starch used for and why?
Undoubtedly, you will have used Maizena, or cornstarch, to thicken creams or to make pudding before. Or perhaps added a bit of flour to a béchamel sauce when making lasagna or moussaka. As it turns out, cornstarch is starch extracted from corn grain, similarly to how wheat flour is derived from wheat grains.
Cornstarch is an example of a starch that finds numerous applications in the food industry. Companies use it as a stabilizer and thickening agent, or most notably to make high fructose corn syrup (HFCS). HFCS is a sweetener, which the industry uses in anything from sweetening juices to soft drinks, sometimes even in bread. It is cost-effective and retains moisture, thereby making it attractive to food manufacturers.
High fructose corn syrup is made by simulating the breakdown of starch into its basic component, glucose, which typically occurs in our digestive system. Once the starch is converted to glucose, food manufacturers will convert some of the glucose into fructose, which is a bit sweeter. We end up with many different ‘types’ of HFCS: HFCS-42 contains 42% fructose and 48% glucose while HCFS-55 contains 55% fructose and so on. HFCS is essentially a glucose-fructose concentration with some water molecules mixed in.
How starch breaks down into sugar and why it matters
When we consume starchy products, they enter our mouths and are immediately confronted with intimidating enzymes in our saliva. Amylase enzymes in our saliva take apart starch molecules. They thereby begin metabolizing it before it even has a chance to reach our stomach. Only upon reaching the small intestine, a subtype of amylase, γ-amylase further breaks down the starch molecules.
The key difference between the amylase in our saliva, α-amylase, and the amylase in our small intestine, γ-amylase, is the method they use to break down starch. α-Amylase randomly cleaves glycosidic links (a specific type of them called α-1,4-glycosidic links) between glucose molecules. This random cleaving produces shorter chains of glucose molecules, called dextrose (polymers of glucose units) and maltose (two glucose units). This produces somewhat digested starch, namely a mixture of dextrose and maltose, which awaits further digestion inside the body.
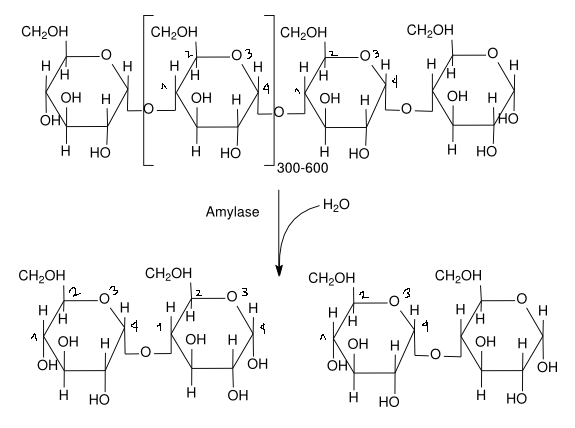
Once inside the intestines, unlike α-amylase, γ-amylase cleaves two different types of glycosidic links (the links that bind glucose molecules together). It cleaves both α-1,4-glycosidic links, which are links between linear glucose molecules, and α-1,6-glycosidic links, which are links where glucose molecules split off into different branches. Once γ-amylase has finished, we are left only with glucose molecules, which the body uses for various processes.
In industry, glucose is often made from starches, which are cheap and abundant, making this more cost-effective. In beer brewing, yeast ferments glucose molecules, which improve the beer’s flavor and richness. These sugars are often added in the form of sugar syrups, which are derived from starch. Sugar syrups are also established ingredients in candies, and seeing as yeast ferments glucose and produces ethanol, they are used to produce ethanol as biofuel, as well.
The experiment
Industry has immensely improved their starch-to-glucose processes over the past millennia. The very enzymes at play in your saliva and intestine are for sale online, and it isn’t difficult to make your own high fructose corn syrup at home. Theoretically it is even possible to make table sugar out of glucose syrup by dehydrating it.
This brings me to my original quest to convert potatoes into cookies. I wanted to use the potato starch and break it down into its simpler sugars: dextrose, maltose and glucose. To do this, I followed the following steps:
1. Extracting the starch from potatoes
2. Breaking down the starch with enzymes
3. Making cookies out of ‘glucose syrup’
If you’re curious not to just read about the theory, but also to see the practice, then stay tuned. I will soon be releasing a YouTube video where I follow this little experiment!
- “Lintnerized starches gel-filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch.” ROBIN et al. 1974.
Coultate, T. (2015). Food: The Chemistry of its Components. In The Royal Society of Chemistry eBooks. https://doi.org/10.1039/9781839169168
Wikipedia contributors. (2025a, July 18). Starch. Wikipedia. https://en.wikipedia.org/wiki/Starch
How is HFCS high fructose corn syrup produced ? (n.d.). Starch Processing Machine. https://www.starchprojectsolution.com/faq/high_fructose_corn_syrup_produce_1170.html
↩︎